INDICATORS
-
Indicators are special type of substances that indicate whether a substance is acidic or basic.
-
The indicators change their colour when added to an acidic or basic solution.
-
Turmeric, litmus, China rose petals (Gudhal), etc., are some of the naturally occurring indicators.
Litmus:
-
Litmus is a purple dye extracted from lichens and is available either in the form of solutions or in the form of strips of paper known as litmus paper.
-
A blue litmus strip when dipped in acidic solution acquires red colour and red litmus acquires a blue colour when dipped in basic solution.
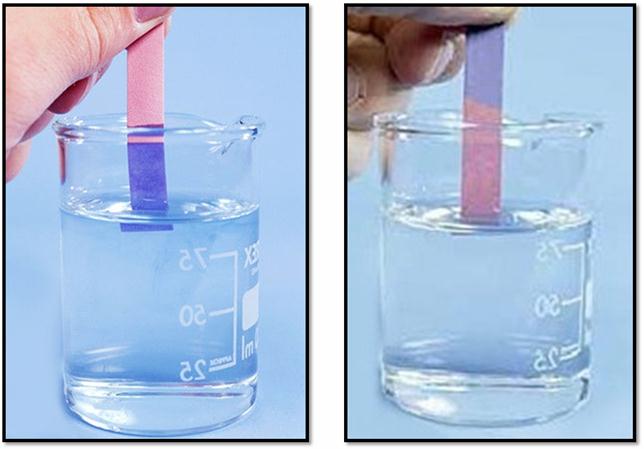
-
In addition to litmus, phenolphthalein and methyl orange are also used as indicators.
Neutral substances:
-
The substances that do not have any effect on the colour of any of the indicators are neither acidic nor basic.
-
These substances are called neutral substances.
-
Some examples of neutral substances are sodium chloride, sugar, etc
Colour change of indicators in acidic, basic and neutral medium
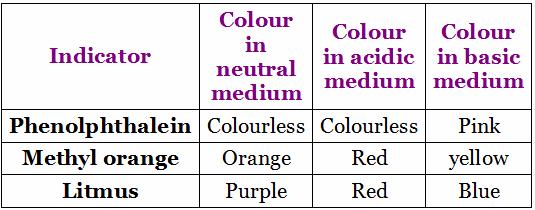
Universal Indicators:
-
Acids or bases can be either strong or weak. In order to measure the strength of acids and bases, universal indicator is used.
-
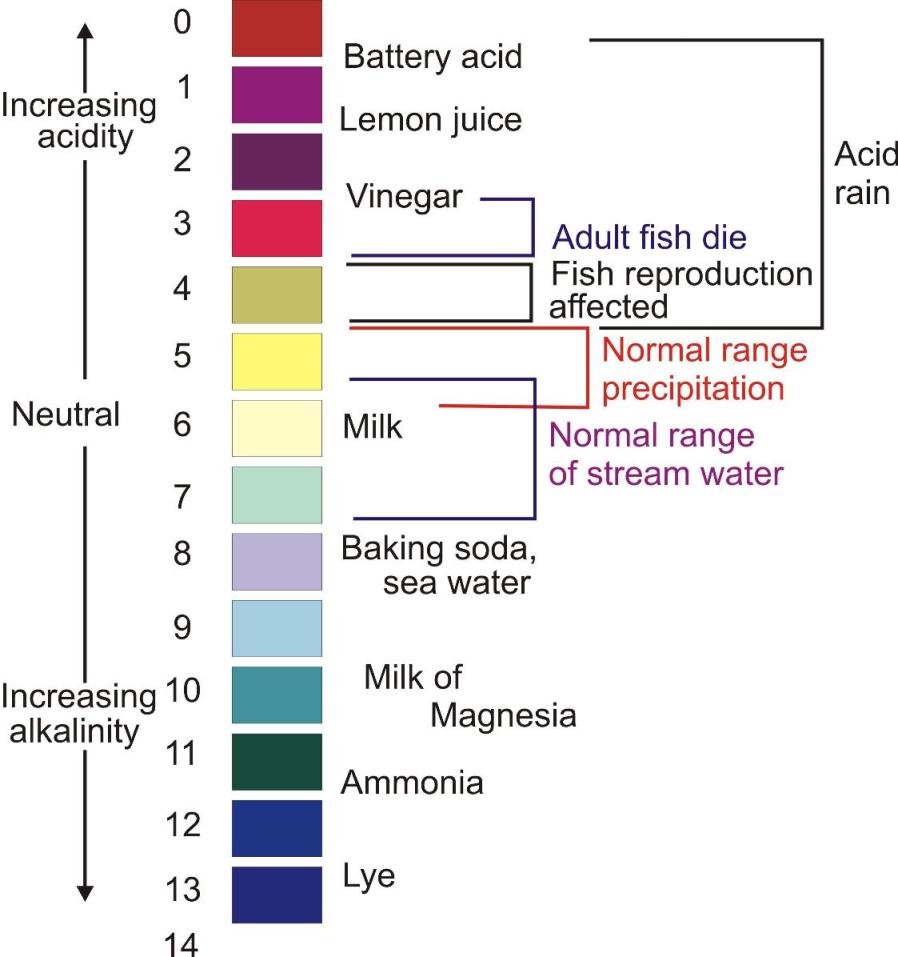
Strengths of acids and bases is given by pH scale.(In German ‘p’ stands for ‘potenz’ meaning power.
-
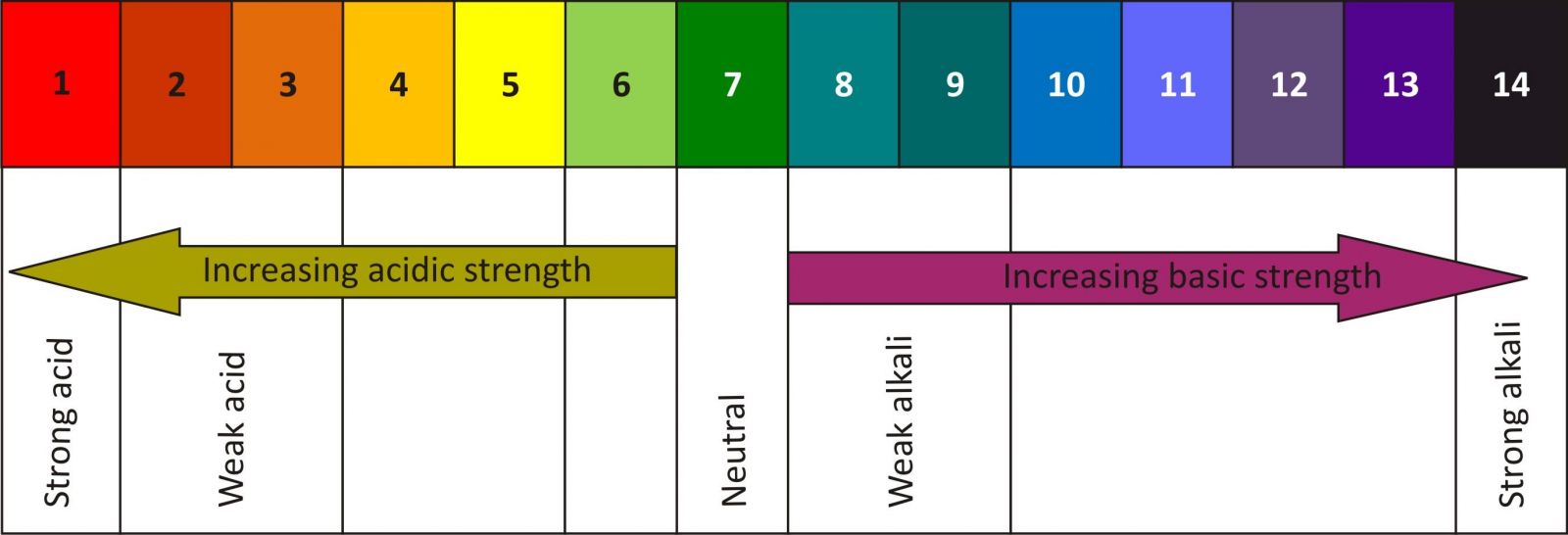
pH scale ranges from 1-14.
pH scale
-
1 is considered as strongly acidic and 14 strongly basic.
pH 7 is neutral.
-
A universal indicator is a mixture of indicators chosen in such a way that it gives a different colour for different pH values.
-
The indicator can be used as a liquid or can be soaked into paper. This paper is called pH paper.
-
When a pH paper is dipped in an acid or a base, the colour obtained can be matched with the chart provided with pH paper.
Natural Indicators:
-
Indicators that can be prepared very easily from brightly coloured parts of plants such as flowers (china rose, rose), roots (beet root), stems (turmeric), and leaves (red cabbage), by boiling coloured parts of the plant e.g., petals in water for some time and straining out the petals.
-
This solution obtained gives different colour in acidic and basic medium.
For example
-
Red cabbage juice will change to deep red with acids, to purple with neutrals, and to green and yellow with bases.



