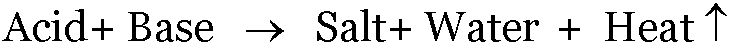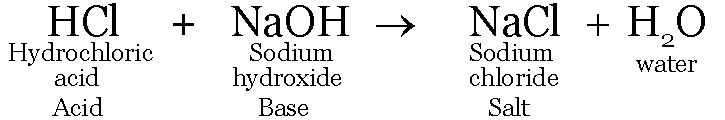NEUTRALISATION
-
When an acidic solution is mixed with a basic solution, both the solutions neutralize the effect of each other.
-
The resulting solution is neither acidic nor basic.
-
In neutralization reaction a new substance is formed which is called salt. Salt may be acidic, basic or neutral in nature.
-
The reaction between an acid and a base is known as neutralization.
-
Salt and water are produced in this process with the evolution of heat.
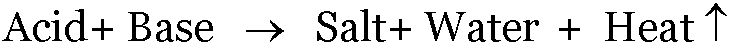
-
In neutralization reaction, heat is produced, or evolved due to which the temperature of the reaction mixture increases.
For example
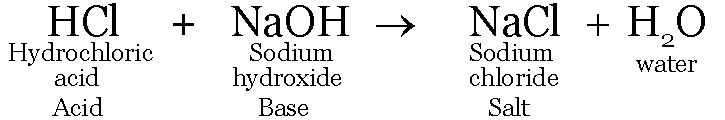
Neutralisations In Everyday Life:
Indigestion:
-
Our stomach contains hydrochloric acid which helps in digestion of food.
-
But too much of acid in the stomach causes acidity.
-
To relieve acidity we take antacids like Eno, Gelusil, etc.
-
It neutralizes the effect of excessive acid.

Ant sting:
-
The sting of an ant contains formic acid.
-
When an ant bites, it injects the acidic liquid into the skin.
-
The effect of the sting can be neutralized by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
Soil treatment:
-
Excessive use of chemical fertilizers makes the soil acidic.
-
Plants do not grow well when the soil is either too acidic or too basic.
-
When the soil is too acidic, it is treated with bases like quick lime (calcium oxide) or slaked lime (calcium hydroxide).
-
If the soil is basic, organic matter is added to it.
-
Decomposition of organic matter produces acids which neutralize the bases present in the soil.
Factory wastes:
-
The wastes of many factories contain acids.

-
If they are allowed to flow into the water bodies, the acids will kill fish and other organisms. The factory wastes are, therefore, neutralized by adding basic substances.