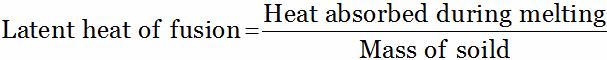LATENT HEAT OF FUSION
-
The amount of heat energy that is required to convert 1 unit mass of a substance from solid state to liquid state at its melting point (at atmospheric pressure) without any change of temperature is known as the latent heat of fusion.
-
Latent heat of fusion of ice is the quantity of heat required to convert one unit mass of ice into liquid water at 0ºC (under normal pressure) is called Latent heat of fusion of ice
-
For water at its normal freezing point of 0ºC, the latent heat of Fusion is 33500 J kg–1. This means that to convert 1 kg of ice at 0⁰C to 1 kg of water at 0ºC, 335000 J of heat must be absorbed by the water. Conversely, when 1 kg of water at 0ºC freezes to give 1 kg of ice at 0ºC, 335000 J of heat will be released to the surroundings.
-
So, particles in water at 0ºC (273 K) have more energy as compared to particles in ice at the same temperature.
Heat energy require to melt ice at its melting point:
Q = MLf
Where Q is the amount of heat absorbed by the solid, M is the mass of the solid and Lf is the latent heat of fusion measured in cal/g (to fuse means to melt).
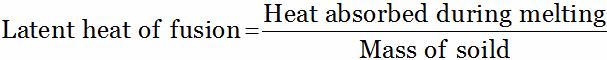
Natural consequences of Latent heat of fusion of ice:
-
Water has one of the highest latent heat of fusion values of all substances and therefore has several applications.
-
Ice cubes are added to cold beverages rather than ice cold water.
Reason:
-
The latent heat of fusion of water is 3.3 × 105 J/kg. That is, 3.3 × 105 J of heat are needed or absorbed to melt 1 kg of ice. The heat required to melt the ice comes from the food or drinks in the beverage. As heat leaves the food, it becomes cold.
-
Preventing Frost Damage
-
When 1 kg of water freezes it releases 3.3 × 105 J of heat energy.
-
Farmers use this principle to prevent frost damage to their orchards and other crops.
-
When a frost is predicted, farmers turn on the water sprinklers. As the water falls on the plants and starts to freeze, heat is released to the surroundings and plants. The heat helps the plants stay warm enough to prevent damage. This only works when the temperature does not drop much below freezing.

-
Melting of Snow in Mountains:

-
The snow on mountains does not melt in one go because it needs a lot of energy to melt (35000 j kg–1). At higher altitudes and high slopes of the mountains, the intensity of heat energy is low. As a result the snow on mountain melts slowly. The slow melting of snow helps us in two ways:
-
It prevents flooding of rivers.
-
Rivers remain full of water round the year.
-
High specific heat and latent heat of water makes the survival of aquatic animals during winters possible:

Specific heat
-
The quantity of heat required to raise the temperature of a whole body by 1 K (or 1ºC) is called its heat capacity)
-
Specific heat capacity of water is much higher than those of all other common substances.
-
For the same quantity of heat and the same mass, water will show the lowest rise or fall in temperature.
-
Lakes and ponds in colder regions do not freeze at once.
-
High specific heat of water and high latent heat of freezing makes water to lose heat energy slowly. This makes only the upper layer of water to freeze. The lower layer of water remains in liquid form due to higher density. This makes the survival of aquatic animals possible during winter.
-
In cold places, the weather becomes very cold as soon as snow starts melting. This is because snow absorbs heat (equal to its latent heat of fusion) from the surroundings.
-
Icebergs can be carried by ocean currents over long distances without melting, due to high latent heat of fusion of ice.
-
It becomes very cold after hail storm.
-
Hail storm: Any thunder storm which produces hail that reaches the ground is known as hail storm.
-
Hail: Hail is a form of solid precipitation. It consists of balls or irregular lumps of ice each of which is referred as hail stone.
-
When the hail stone melts it takes heat energy equal to its latent heat of fusion thus cooling the weather.